
Quality management should be a holistic approach. Process, product and people are the three key elements of a company’s quality system and all of them need to be focused on for an overall improvement in performance.
Process
Process audits and statistical process control are two important tools that practitioners can use for process monitoring. Audits are good tools for monitoring a system and following up with corrective actions in deficient areas. The ISO 9000 quality management audit and the ISO 22000 food safety audits are beneficial tools; however, these audits are broad based and may not provide the required depth of sampling for close process monitoring.
A process audit that focuses on the actual company process and covers all areas of a company’s operations is a better diagnostic tool to assess the health of the company’s processes. Figure 1 shows a process audit sample from a company that has a large presence in an emerging market.
| Figure 1: Sample of Process Quality Audit Checklist | |||
| Item | Check | Sample | Remarks |
| Raw materials | |||
| Certificate of analysis from the suppliers | 10 | Report findings | |
| Special testing | 30 based on the 5 atypical testing categories | ||
| Raw material rejections percentage vs. receipts | Monthly data | Target less than 3 percent | |
| Packing material | |||
| Certificate of analysis from the suppliers | 10 | Report findings | |
| Testing | Less than 5 percent deviation | ||
| Corrective actions on complaints | |||
| Finished goods | |||
| Testing | 30 strategic customers | Report findings | |
| Shelf-life issues | In compliance | Less than 5 percent deviation | |
| Customer specifications | |||
| Microtesting | |||
| Methods | |||
| Equipment validation | 5 | ||
| Reagent validity | |||
| Laboratory validity | |||
| Sensory | |||
| Global procedure adhereance | All | Compliance | |
| Sensory evaluation of room conditions | |||
| Training of new persons | |||
By doing audits on a periodic basis, any gaps in the process will be uncovered and successfully closed to improve the process.
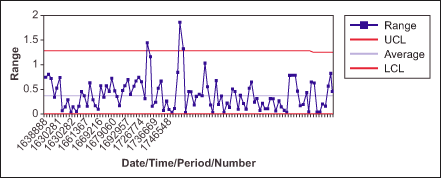
Statistical process control is another useful tool to monitor the performance of a process. With this method, practitioners plot and study the average values (X) or range (R) values. Stable processes vary within control limits, which are based on past performance, in a set pattern.
Process variation with respect to the product or service specification is monitored through two process capability indices, Cp and Cpk.
Cp gives the process variation with respect to the specification limits, which represent the area of acceptable performance, based on the formula,
![]()
For a stable process, Cp is greater than or equal to 2.
Cpk indicates the process variation with respect to control limits as well as the target value, and is denoted by the formula,
![]()
For a stable process, Cpk is greater than or equal to 1.33.
Figures 2 and 3 show the average and range charts with Cp and Cpk values for the salt content in a product manufactured by the company. The values for both Cp and Cpk are lower than the target (Table 1), indicating the process needs to be further improved.
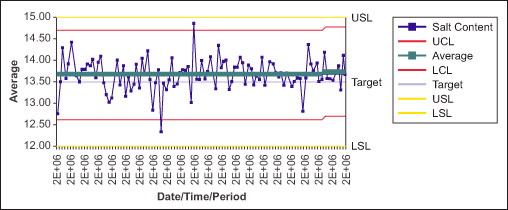
| Table 1: Process Capability | |
| LSL in theory | 12.00 |
| LSL in theory | 15.00 |
| Cp | 1.40 |
| Cpk | 1.24 |
Product
Of course, monitoring product quality is important, especially when the product is consumed. A robust system should have product inspection mechanisms to address the following requirements:
- The evaluation is completed based on customer and international standards.
- The customer requirements are constantly monitored and product quality is updated based on feedback.
- Customer complaints and nonconformance are addressed in a systematic manner using Six Sigma to identify root causes and corrective actions.
Companies should engage in revising product specifications on a continual basis based on customer and market requirements.
Design of experiments (DOE) is a particularly useful tool in improving product quality. The following example is an illustration of the application of the tool.
Situation: Product A is a food colorant and an additive that the company manufactured by the controlled heating of a raw food material in the presence of a catalyst. The color of the product was the key quality parameter and needed to be maintained within a specification range.
Problem: The main issue was found to be reproduction of the color value. The specification was 20 to 21 color units. About 20 percent of the batches had either less or more than this specification. Another batch was made and the two batches were mixed to meet the specification. This led to delays in production as well as quality costs due to rejections.
Objective of DOE: To vary the two critical input parameters – catalyst quantity and time of reaction – to arrive at the optimum conditions.
DOE Study: This was a two-factorial experiment with catalyst quantity and time at two levels, high and low. Four experiments were performed and practitioners studied the output – color value (Table 2). Practitioners found the product met the target when the catalyst and time were at the lower levels.
| Table 2: Factorial Experiments | ||
| Reaction Time | Quantity of Catalyst Used | Color Value |
| 46 | 5 kgs | 25.7 |
| 36 | 5 kgs | 19.4 |
| 36 | 6 kgs | 18.9 |
| 46 | 6 kgs | 20 |
People
People are an important part of the quality system. They need to be assessed and trained on the latest requirements of process and product quality as required by the customers and market. People alignment and development for job efficiency can be done in a systematic way through evaluating workers’ skills, training to fill the identified gaps and empowering employees.
Skills Matrix
Identify the skills required for a job. The skills need to be categorized as technical and managerial. A matrix can be developed as shown in Figure 4.
| Figure 4: Skills Matrix | ||||
| Competency | Employee 1 Requirement | Employee 1 Status | Employee 2 Requirement | Employee 2 Status |
| Technical | ||||
| Basic food chemistry | Medium | Medium | Medium | Low |
| Citrix system | High | Medium | High | Medium |
| SAP | High | Medium | High | High |
| Customer complaints | High | High | Medium | Medium |
| Micro-testing | Medium | Medium | High | High |
| Analytical testing | High | High | High | High |
| ISO system | Medium | Low | Medium | Low |
| HACCP principles | Medium | Low | Medium | Low |
| Sensory training | High | High | High | Medium |
| Using statistics | Medium | Low | Medium | Low |
| Allergens | Medium | Low | Medium | Medium |
| Managerial | ||||
| Time management | High | High | High | Medium |
| Subordinate development | High | Medium | N/A | N/A |
| Problem solving | Medium | Medium | High | Medium |
| Decision making | Medium | Medium | High | High |
*Red indicates areas that are in need of improvement
Competency Training
Using the matrix, it is possible to determine what competency training needs to be organized. It can be done internally or externally and should be a time-bound program. Assessment of skills should be done on a periodic basis to measure improvement and see if any retraining is required.
Empowerment
It is important that the people assigned to a job are fully empowered – they must have the authority to carry out the assigned responsibility.
Using the 3P Approach Successfully
One large manufacturing plant that utilized these principles between 2006 and 2007 was able to reduce customer rejection rates by more than 40 percent and implement an employee succession planning process in the quality department.
When properly used, this approach for focusing on the three key areas of quality management – process, product and people – will help companies move toward Six Sigma quality with fewer defects and more satisfied customers.